

The first is based on the benzalkonium tetrathiocyanatocobaltate(II) ion pair ( 5). It is worth mentioning two studies designed to obtain cobalt(II) ion-selective membrane electrodes (5,6). The measurement of cobalt(II) with and ion-selective electrode has hitherto been possible only in an indirect way with a copper(II) ion-selective membrane electrode (Orion, Model 94-29) based on the interaction between cobalt ions and the EDTA complex of copper(II) ( 4) Several unsuccessful attempts ( 1–3) have been made to produce cobalt ion-selective membrane electrodes. COSOFRET, in Membrane Electrodes in Drug-Substances Analysis, 1982 Discussion and comments Flow cytometry and PI/Annexin V results indicated that the anti-tumorigenic activity of SKLB325 is induced by the upregulation of p53 and consequently the cell death (apoptosis) mechanism. These findings support the hypothesis that JMJD6 inhibition exerts a strong therapeutic effect in mouse models. Treatment with this compound reduced tumor volume and prolonged survival compared with the two control groups in a peritoneal carcinomatosis model in BALB/c nude mice. In ovarian cancer, SKLB325 inhibits JMJD6 expression and cell proliferation in vitro in a dose-dependent manner. The only compound that selectively inhibits JMJD6 is SKLB325 ( 14), whose activity was very recently described (2019). 6.3) inhibit both KDM and RDM activity in vitro, but with controversial effects in vivo. A recent study revealed that 2,4-PDCA ( 45), NOG ( 39), deferoxamine, JIB-04 ( 46), succinic acid disodium salt hexahydrate, fumaric acid, α-hydroxyglutarate (2-Hg 36), nickel (II) sulfate heptahydrate, cobalt (II) chloride hexahydrate, IOX-1( 48), CPI455, and KDM5-C49 ( Fig. However, since it shares structural homology with the JmjC family, this enzyme is inhibited in vitro by some compounds already described in the literature as broad-spectrum JmjC KDM inhibitors. To date, few selective compounds inhibiting JMJD6 activity have been developed.
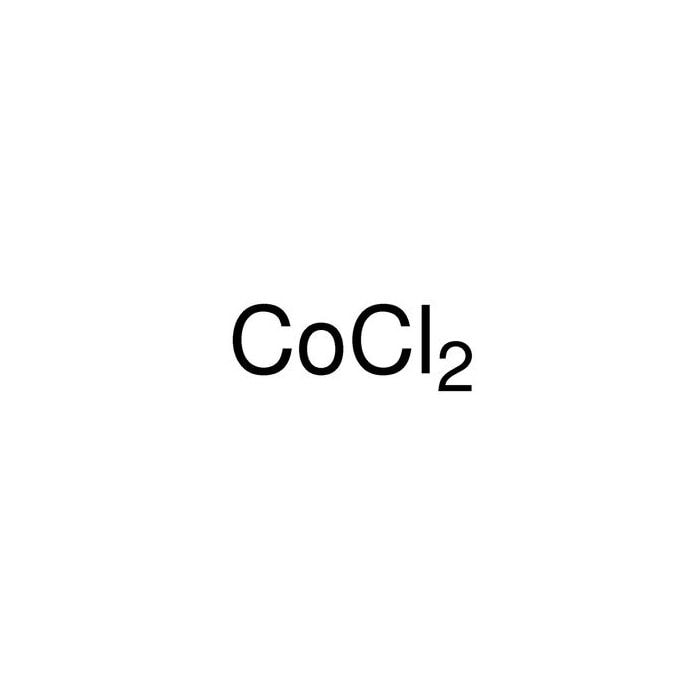
Lucia Altucci, in Histone Modifications in Therapy, 2020 6.2.2.2 JMJD6 inhibitors Cobalt sulfate: monohydrate: dissolves slowly in boiling water heptahydrate, soluble in water, slightly soluble in ethanol.įederica Sarno. Cobalt oxide: Insoluble in water, soluble in acids or alkalis. Cobalt chloride hexahydrate: soluble in water, alcohols, acetone, ether, glycerol.

Cobalt chloride: soluble in water, alcohols, acetone, ether, pyridine, glycerol.

Cobalt carbonate: - Almost insoluble in water, alcohols, methyl acetate. Cobalt sulfate: 155Ĭobalt acetate: - Soluble in water, alcohols, diluted acids, pentyl acetate. The following data are from The Merck Index (8th edition).Įlemental cobalt is a hard, silvery grey metal whereas Co sulphate occurs as red to lavender crystals.Ĭobalt acetate: mw: 177.03-Ligth pink crystals-Soluble in water, alcohols, diluted acids, pentyl acetate.Ĭobalt carbonate: mw: 118.95-Rod powder or rombohedral crystals-Almost insoluble in water, alcohols, methyl acetate.Ĭobalt chloride: mw: 129.85-Pale blue hygroscopic leaflets turns pink upon exposure to most air.Ĭobalt chloride hexahydrate: Pink to red slightly deliquescent, monoclinic, prismatic crystals.Ĭobalt oxide: mw: 74.94- Powder or cubic exagonal crystals-Color from olive green to red depending on particle size-Insoluble in water, soluble in acids or alkalis.Ĭobalt sulfate: Red to lavender dimorphic, orthorhombic crystals.Įlemental cobalt 58.933. Co can occur in four oxidation states (0, +2, +3, +4).


 0 kommentar(er)
0 kommentar(er)
